.jpg)
Do not hesitate to contact me on the forum https://www.hairrestorationnetwork.com (my nickname is arthurSam) : Click here to send me a message !
(Updated March 13, 2023: addition of two videos on exosomes: "Extracellular vesicles characterization and therapeutic potential" & "Discussion and Q&A with the audience")
(Updated January 10, 2023: addition of information relating to the microRNA mir-122-5p contained in the ASCE+ HRLV product)
(I also created a topic here on the forum https://www.hairrestorationnetwork.com/)
.jpg)
The study presented in the video above by Professor Inigo DE FELIPE Y GARATE shows that the addition of ASCE+ HRLV exosomes to standard treatment {Dutasteride 0.5mg/day + oral Minoxidil 5mg/day} provides a benefit of 46% for the effectiveness of the treatment over 1 year (C: control group without exosomes / EXO: group with exosomes):
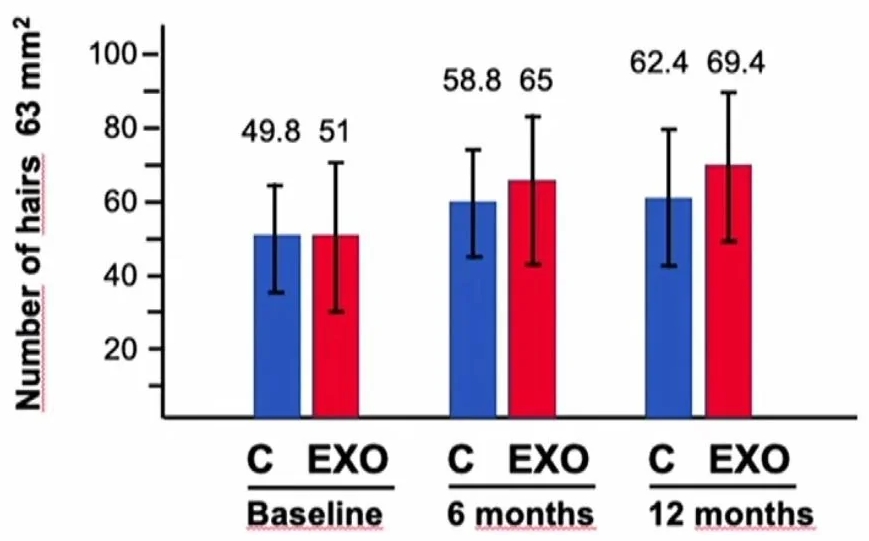
So I started studying exosomes to understand how they could be useful to me and I have been taking them for a few weeks now...
Following my 6500 FUT hair transplant without shaving (which is equivalent in term of hairs to a 3000 FUE grafts - my choice fell on FUT because it is this type of graft that makes it possible to maximize the grafts and to have the most dense and solid grafts and especially the doctor could do it for me without shaving!) more than a year ago made by Paul Benet (see ici the photos with the nickname EricLaurent - my special transplant account), I am interested in treatments to fight against the progression of my baldness.
As I still had some primitive losses following my transplant (a transplant does not stop alopecia from evolving!) , I started by taking 0.025% finasteride topically, 3 times a week (in addition to the 5% minoxidil that I have been taking for several decades). This allowed me to stop my fall but I had to stop the treatment due to the appearance of side effects: testicular pain, prostate pain, frequent urination...
So I then tested several drugs topically, including dutasteride based on a siliconized liposomal gel such as Xyon, which I bought at the Parati pharmacy (the constitution of Parati gel is the same as that of Xyon). Since this formulation was normally supposed to not penetrate the bloodstream but only into the follicles, I was quite confident... but unfortunately, again, I had side effects quite quickly and had to stop my treatment.
Unfortunately these pains did not stop immediately, but even continue a little today! That's when I consulted Dr. Mousseigne for his opinion and he told me that I had PFS (Post Finasterid Syndrome). He then offered me to take a treatment that was banned in France based on steroids but that I could find in Morocco. If I could get the drugs through contacts in that country, he was ready to help me dose and use them. However, I preferred not to take these steps because this medication is very dangerous.
During this interview, he also wanted to look at my transplant and he very much appreciated Mr. Benet's work. He told me that my transplant was very successful :-)
I am of course very satisfied with my transplant, which Mr Mousseigne said was very successful.
But you have to think long term and protect your hair, even after being transplanted!
So I then started a topical treatment {Fluridil + CosmRNA} in June 2023, about 1 year after my transplant (7.5 months as of the day I am writing these lines) to fight against the action of DHT, and of course while still being on 5% topical minoxidil. This treatment stopped my hair loss and I no longer have any primitives that fall in the shower anymore!
It is extremely positive! In addition, It seems that since taking {CosmRNA + Fluridil} small hairs are starting to appear (see my post here and here and here) ... So now I hope to fortify them with ASCE+ HRLV exosomes from ExocoBio.
So I'm going into exosomes hoping to gain a bit more density. I no longer have hair that falls out and so it seems that my baldness has stabilized at the moment with my treatment.
On the international-hairlossforum.com forum, I had two accounts: ArthurSam2 to discuss hair loss treatments, and EricLaurent to discuss the results of my hair transplant.
At the time, I chose to use two different aliases because I noticed that all users who presented results of transplants not made by Belgian doctors or by Mousseigne were systematically denigrated.
I had also noticed that they are very aggressive towards users who have been grafted with techniques other than FUE... yet, large reputable clinics continue to carry out FUT, such as Hattigen for example (ie, see this video) ! As long as you want good density in a single session, this is what is recommended if you accept the linear scar in the back of the head. But this technique is much more delicate than FUE and requires a great deal of experience that few doctors continue to have...
In fact, I discussed this with Mr Bisanga at the time. He told me then that FUT was a very effective technique but that with the increasing number of requests to perform FUE, he had focused more on FUE and that he no longer had staff to carry out FUEs. Here is an excerpt of his response:
In terms of pricing, FUT requires twice the number of surgical team/techs than FUE and so is no longer the more economimcal opton. The reality of this, is that with FUE “leading” the market over FUT, clinics no longer require the same quantity of technicians as they once did as the vast majority of patients decide to proceed with FUE surgery. When FUT is scheduled, more technicians are required, meaning more expense to be covered, which will be absorbed into the cost of surgery for the patient, oftentimes meaning that FUT may now present a higher price than FUE, and is no longer the more economical option, which in turn results in FUT becoming even less attractive to many.
In addition, there had been legal problems between the forum administrator and Paul Benet and so I did not want to be denigrated from my first message regarding my experiences with hair loss treatments! So I preferred to put anonymity between my hair transplant and my experiences with treatments.
But one user finally noticed my double account and started harassing me on the forum.
All the posts I created on the forum international-hairlossforum.com are polluted by the user Finn and his cronies who are harassing me and who forced me to be banned because of this double account. These guys are real collaborators don't trust them!
All these users who harass me do it because my transplant was not done by their grafter “Mousseigne” and they cannot stand the high quality of my transplant, which Mr. Mousseigne rightly said was done very well (at least, this gentleman is honest... not like all those users who harass me on the forum...).
These users who frequent the international-hairlossforum.com forum systematically discredit all those who do not transplant with Mousseigne
They are certainly paid by this gentleman to do that!
These users started by writing all over the international-hairlossforum.com forum that my photos were stolen or faked:

(translation : ArthurSam/EricLaurent uses photos of another person to promote his transplant and the effects of CosmRNA)
Then these users even registered on the international forum https://www.hairrestorationnetwork.com which I also frequent to discredit and pollute all my messages.
Finn and the administrator of the international-hairlossforum.com forum even tried to make the administrator of the English forum believe that my transplant was done by Mousseigne and not by Paul Benet, all in order to get me banned:


(translation : I would probably do a post again, in addition I have the assertion that the person from whom he was taking his photos had his transplant at Mousseigne and not at Paul Benet's, beware of him)
So you can see that even the administrator of the international-hairlossforum.com site is taking part in this whole masquerade!
These same users also sent fake messages on reddit, in agreement with the administrator of the international-hairlossforum.com forum by stealing my photos posted on the hairrestorationetwork.com forum and posting them with defamatory remarks:

You can see how hateful and harmful these users who have been transplanted by Mousseigne are when you don't go through the same doctors as them!
Once again, they encourage newcomers to the forum to be transplanted by Mousseigne and they are certainly paid by this man!
Finn is even now continuing his investigation among the Germans... :
(translation :people don't get fooled by this character he's lying, everything he says is wrong. Many of his photos are found elsewhere on the internet I hope one day to find the real owner so that he can file a complaint.
I will go and see on the German forums maybe I will find what I am looking for.)
Come on, let's get serious!
Two videos (in English) on exosomes, their principle of operation and a question-and-answer session relating to their use:
 |
 |
| Extracellular vesicles characterization and therapeutic potential | Discussion and Q&A with the audience |
So here is a summary of what I have been able to read about this field. My aim was to understand what exosomes are and how they might be able to help me in the fight against alopecia. I wanted to get an idea of the effectiveness that the ASCE+ HRLV product (which I chose to use) can have, and try to understand how it can improve my sad condition...
The ASCE+ HRLV product seems to be very common in clinics and therefore of good quality. There are much cheaper exosomes like Hanheal Hair but I have big doubts about the quality of this product...
You will see that after doing research in order to fully understand the role that exosomes can have on alopecia, I am unfortunately a bit divided on the ASCE+ HRLV product in terms of the theoretical effectiveness that it could have. But I'm going to start anyway because there seems to be good feedback from dermatologists who use it in mesotherapy... And besides, alopecia is a very complex process. Not being a doctor, I would also like to seek the help of members of this forum to contradict, or on the contrary confirm, my ideas and my remarks.
The routine I plan to follow with these exosomes will be as follows:
For mesotherapy, I use the mesogun dermajet 300 with mutineedles 5-pin 1mm needles:


Procedure during a session:
Do not touch the scalp for the next 4 hours.
Do not apply anything, shampoo or exercise for the next 12 hours.
To follow the changes, I will target specific areas of my scalp and I will post images every month.
Of course, I am only posting here for informational purposes, I do not know if this product will work and therefore I am not making any recommendations on its purchase and use!
Summary:
Sources :
The AR/miR-221/IGF-1 pathway mediates the pathogenesis of androgenetic alopecia
Biological Function of Exosome-like Particles Isolated from Rose (Rosa Damascena) Stem Cell Culture Supernatant
Targeting Wnt/β-Catenin Pathway for Developing Therapies for Hair Loss
Dihydrotestosterone-Inducible Dickkopf 1 from Balding Dermal Papilla Cells Causes Apoptosis in Follicular Keratinocytes
Perspectives on miRNAs Targeting DKK1 for Developing Hair Regeneration Therapy
The Hair Follicle as a Dynamic Miniorgan
Mesenchymal Stem Cell-Derived Exosomes and MicroRNAs in Cartilage Regeneration: Biogenesis, Efficacy, miRNA Enrichment and Delivery
Mesenchymal Stem Cell-Derived Exosomes Promote Fracture Healing in a Mouse Model
Adipose Mesenchymal Stromal Cell-Derived Exosomes Carrying MiR-122-5p Antagonize the Inhibitory Effect of Dihydrotestosterone on Hair Follicles by Targeting the TGF-β1/SMAD3 Signaling Pathway
Happy reading!
What are exosomes?
The discovery of extracellular vesicles, or exosomes, dates back to the 1940s. Researchers only started to be interested in them in the mid-2000s when they realized that they allow the exchange of information between cells. There were more than 3000 publications on this subject in the years 2018 and 2019... the race to commercialize therapies based on the use of exosomes is therefore on. The four main start-ups in this field are Codiak Biosciences, Exosome Diagnostics, Evox Therapeutics and ExocoBio. They received over $385 million in investment. There have also been numerous agreements that have been created between start-ups and large pharmaceutical companies.
Exosomes are a class of extracellular vesicles (EVs — Extracellular Vesicles) of nanometric size (30nm to 200nm), released by virtually all eukaryotic cells (cells that have a nucleus linked to a membrane - the cells of our body). Exosomes can be extracted from stem cells and can have numerous therapeutic applications. These therapeutic effects result from a particular form of information transmission between cells: paracrine signaling. Indeed, these signals emitted by the cells induce changes in the cells nearby, which alters their behavior. These signals are transmitted at a short distance, in contrast to the signals transmitted by the endocrine system with hormones. Researchers have shown that exosomes play a very important role in the effects associated with paracrine signaling.
Once exosomes are extracted from the stem cells and purified, they are “loaded with cargo.” This cargo can then be transferred to other cells and therefore change their cellular behavior. It contains the following materials:
Lipids play the role of storing the energy that the cells of our body need to live.
Proteins are macronutrients that form the basis of every living organism. They are synthesized in the cell at the level of the ribosome (which is located outside the nucleus), from the information encoded in the genes (DNA is stored in the nucleus) that determines the order in which the amino acids will be sequenced. Post-transcriptional modifications may occur once the protein has been synthesized, which may have the effect of altering its physical or chemical properties.
Numerous proteins, resulting from the expression of specific genes, are involved in the regulation of physiological events in the skin and hair. We'll come back to this when we study how hair is formed.
Messenger RNAs are copies of a region of DNA that are kept inside the cell nucleus. Since protein-synthesizing ribosomes are outside the cell nucleus, when a cell needs a protein, messenger RNA is exported outside the nucleus and joins the ribosomes where it allows the synthesis of the requested protein.
The rate of transcription of genetic information from DNA to messenger RNA is controlled by proteins called transcription factors. Their role is to activate or deactivate genes so that they are expressed at the right time and at the right level in the body's desired cells. These transcription factors therefore have an important role in cell division, cell growth and cell death. There are 1500-1600 transcription factors in the human genome. These transcription factors can work alone or with proteins.
Non-coding RNAs correspond to all RNAs resulting from DNA transcription but which will not be translated into proteins by the ribosomal machinery. However, they are involved at numerous scales in the regulation of protein synthesis and/or the maturation of other RNAs. Among them, we find, among others, microRNAs (miRNAs).
MicroRNAs are therefore non-coding RNAs that control gene expression at the post-transcriptional level. MicroRNAs regulate gene expression by pairing with target messenger RNAs to which they are partially complementary. They then inhibit the expression of their target genes, causing the blocking of protein translation or their degradation.
Many miRNAs have been identified as being involved in the regulation of physiological events in the skin and hair, such as the regulation of keratinocyte differentiation or in the mechanism of senescence. Some have even been identified as being involved in the development of skin conditions such as psoriasis. We will come back to this point in detail later.
Note that in the category of non-coding RNAs we also find small interfering RNAs (siRNA — Small Interfering RNA). Just like miRNAs, these small interfering RNAs are capable of regulating genes because interference with a specific messenger RNA leads to its degradation and to the reduction of its translation into protein.
Exosomes extracted from mesenchymal stem cells: MSC exosomes
Exosomes extracted from mesenchymal stem cells (MSCs exosomes — Mesenchymal stem/stromal cells), present for example in the mesenchyma (tissue) of the embryo, umbilical cord blood, umbilical cord blood, bone marrow or adipose tissue, seem to be the healthiest for therapeutic use. These MSCs exosomes can also be sterilized while MSC stem cells cannot. MSCs exosomes are considered safe in the field of stem cell treatments. MSCs exosomes have been used as alternatives to MSCs stem cell treatments in many areas: neurological, cardiovascular, immune system, kidney, musculoskeletal, hepatic, respiratory, respiratory, vision, vision, skin diseases and cancers.
The origin of MSC exosomes plays a role in the quality of the therapeutic effect
MSC stem cells have properties of differentiation and self-renewal through cell divisions. These stem cells can be extracted from several types of tissue or liquid: adipose tissue, bone marrow, dental pulp, placenta, umbilical cord, synovial fluid, amniotic fluid or even Wharton jelly.
Depending on their origin, MSC stem cells can be differentiated into various types of cells such as adipocytes (found in adipose tissue), chondrocytes (found in cartilage), osteoblasts (young bone cell) and myocytes (muscle fibers). MSCs also have immunomodulatory properties that regulate various cells that play a role in the immune response. This is the reason why MSCs have been studied so fervently over the last ten years.
Since the characteristics and functionalities of MSCs vary according to their origin, the result is that the same is true for the exosomes extracted from them. Research has shown, for example, that exosomes derived from adipose tissue stem cells (MSC-ASC exosomes) may have a therapeutic effect on Alzheimer's disease but would have contradictory benefits on cancers. The origin of exosomes therefore plays a role in the type of disease targeted.
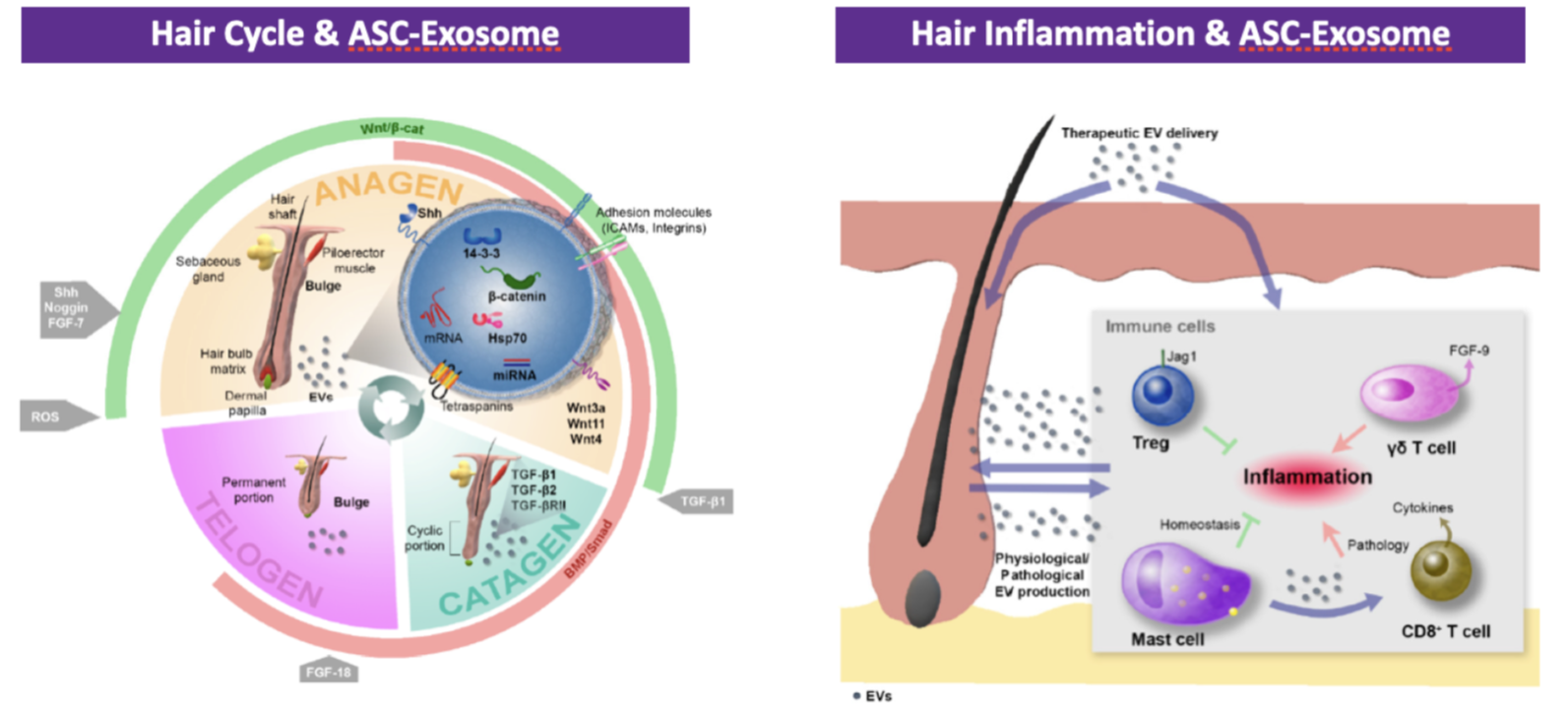
The hair cycle
The hair life cycle is a dynamic and complex process, composed of a rapid growth phase that lasts for a long time (anagen phase, lasting 2 to 8 years), a phase during which the follicles regress (catagen phase, lasting 2 to 3 weeks) and a phase of rest (telogen phase, lasting about 3 months). During the re-triggering of a new anagen phase, a new follicle is created and expels the old follicle (we also speak of an exogenous phase):

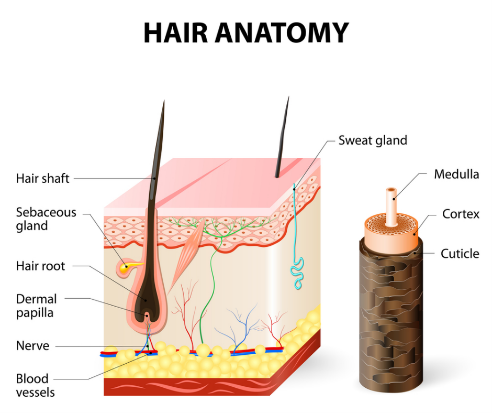
Anatomy of hair
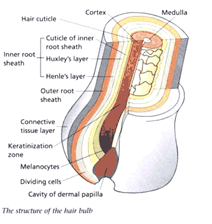
A mature follicle (anagen) can be divided into a visible upper portion and a lower portion that is perpetually remodelled during cycles. It is in the lower part that the factory that governs the formation of the hair is located, in the bulb. This is where the keratinocyte cell matrix (composed of amplified cells derived from follicle epithelial stem cells) and the hair pigmentation unit are located. When this matrix is activated, cells proliferate and differentiate in the region around it. Their number then determines the size of the bulb and the diameter of the hair.
When matrix cells stop proliferating and differentiating, they will have generated various cell lines in the hair shaft and inner epithelial sheath (IRS — Inner Root Sheath). The outer epithelial sheath (ORS — Outer Root Sheath) is an extension of the epidermis and contains a large number of cells that play specific roles in the life of the follicle.
In the bulb is also the dermal papilla, which consists of mesenchymal cells called dermal papilla fibroblasts. Fibroblasts in the dermal papilla generate signals in the matrix causing the proliferation, migration and differentiation of cells in this matrix, which are essential for the growth of the hair follicle. The dermal papilla regulates the size of the bulb, the diameter of the hair and its length as well as the duration of the anagen phase.
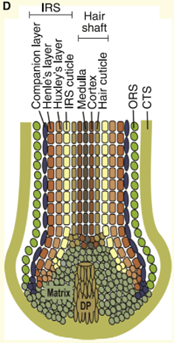
Embryonic follicle development
The essential prerequisite for the development of a follicle is molecular communication between the cells of the epidermis and those of the underlying mesenchyme tissue of the dermis. These communications are organized according to transduction channels, and there are several types of them. Signal transduction is the mechanism by which a cell responds to the information it receives, by chemical agents or other signals (voltage,...). It controls a cascade of secondary signals, internal to the cell (“signalling”) or external (for example an action on other cell types via interleukins), and internal cellular processes (metabolism, cell cycle, mortality, etc.).
The vast majority of transduction pathways involve the attachment of molecules (called ligands) to cell receptors that trigger events inside the cell. Among the molecules that can attach to receptors, there are for example a large number of growth factors (proteins that regulate the manufacture or growth of certain cells), cytokines (a substance secreted by cells of the immune system so that they can communicate with each other), neuropeptides (a hormone that has a neuromodulatory function) and hormones (a chemical substance produced by a gland and which act on the development of an organ), which in part are produced by the follicle itself. The follicle is therefore very sensitive to these various molecules:
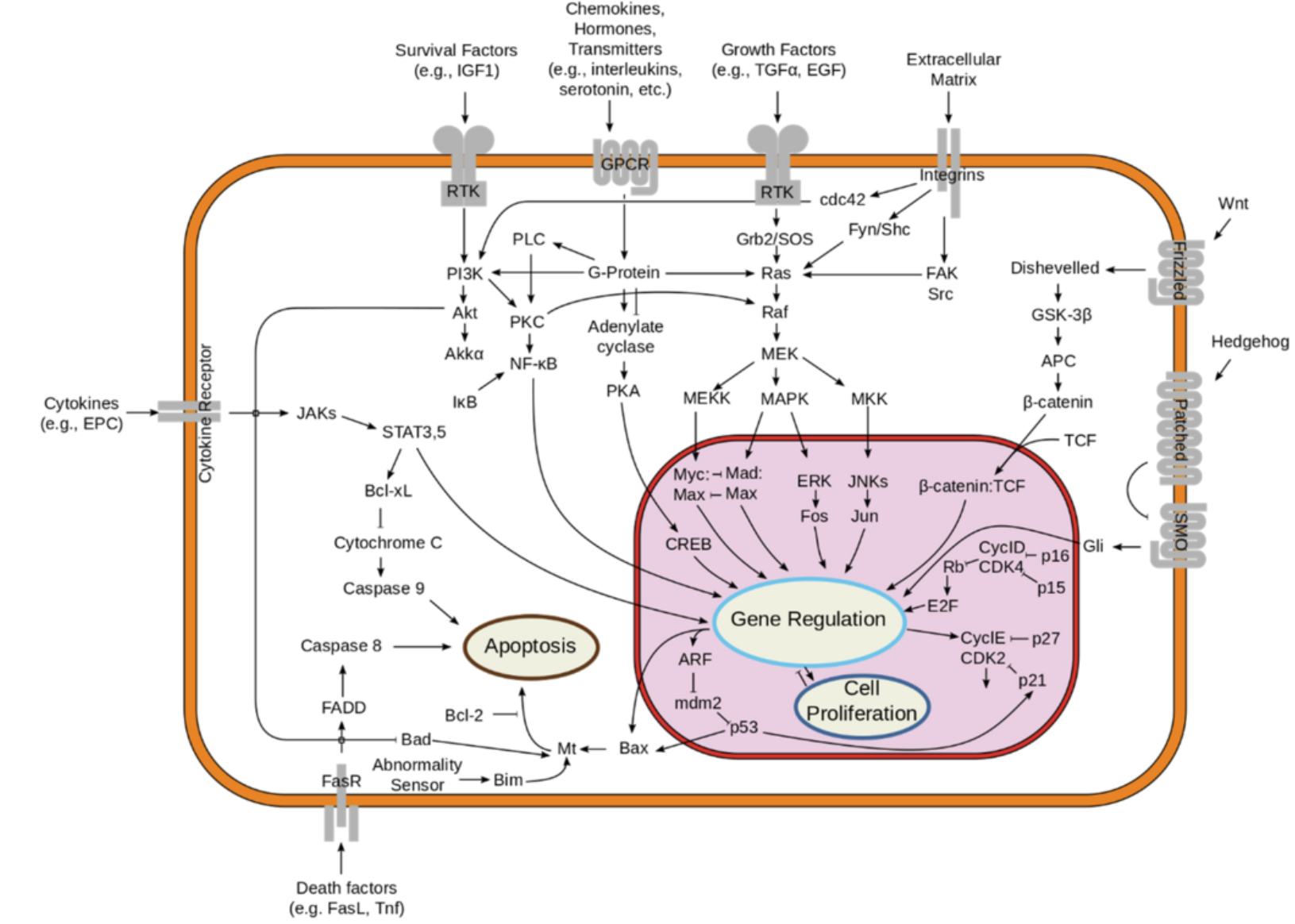
Among the molecules secreted by the transduction pathways that interest us in the case of communication between cells in the epidermis and those of mesenchyme tissue in the dermis, we find the molecules involved in the following families of signaling pathways:
The formation of a hair, which occurs mainly during embryonic life, is induced by a specific combination of these various signals. It has three phases: induction, organogenesis and cytodifferentiation.

The induction phase (steps 0 and 1):
Although hair development mainly occurs during embryonic life, the signaling pathways implemented there also play a very important role in hair regeneration in adults during their life cycle. Indeed, these signaling pathways regulate interactions between epithelial cells and mesenchymal cells in follicles. For example, there are the hedgehog pathways (via the ssh protein), notch, TGF-beta and the Wnt pathway.
The follicle cycle
Once the first postnatal follicle is formed, it begins its life cycle:
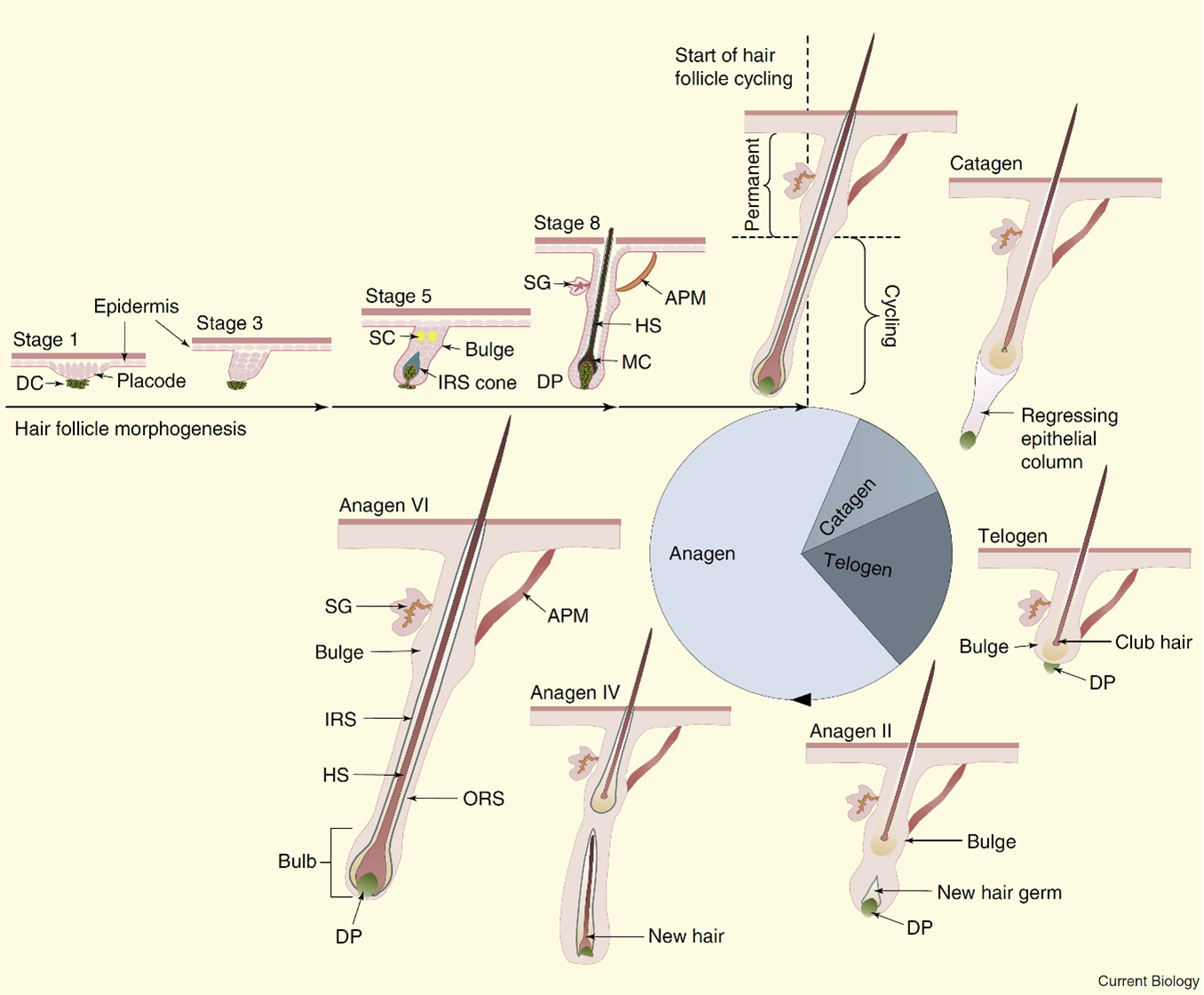
During the anagen phase, the keratinocyte matrix resulting from progenitor cells (of transient amplification) derived from the epithelial stem cells of follicles contained in the bulb, proliferate intensively and generate numerous epithelial cells. This proliferation is activated by the signals generated from the cells of the dermal papilla.
During the catagen phase, two-thirds of the base of the follicle regresses mainly by apoptosis (self-destruction) of the keratinocyte cells that make up the outer layers of the hair, while the stem cells contained in the bulb escape apoptosis. There is also a narrowing of the epithelial canal, which has the effect of separating the dermal papilla from the bulb.
When the catagen phase is over, the follicles go into dormancy (telogen phase). This phase usually lasts longer than 3 weeks and gets longer with age. During this phase, the dermal papilla remains close to the bulb, which allows a very strong interaction between the stem cells contained in the bulb and the dermal papilla. The dermal papilla plays an essential role in the activation of stem cells and the initialization of a new cycle.
Although there are strong correlations between the molecules that will participate in the development of a follicle at the embryonic stage and those that will participate in the renewal of a follicle during the life cycle of the hair, some differences exist. For example, some members of the TGF-beta signaling pathway and some growth factors have opposite functions during embryonic follicle development and renewal: while they stimulate their embryonic development, it stimulates the catagen phase in mature follicles. Other signals, such as those from the notch or VDR signaling pathways (which is controlled by vitamin D), are not essential for follicle development but are essential to induce the anagen phase.
Some molecular mechanisms that dictate the dynamics of these various phases are known. For example, the anagen phase is activated by Wnt/beta-catenin, BMP antagonists, and Shh. However, proteins like TGF-beta1, TFG-beta2, BMP2/4, and TNF-alpha cause the catagen phase.
IGF-1 (Insulin-like Growth Factor One) growth factors, HGF hepatocytes, vascular endothelial growth factors (VEGF) and FGF-type growth factors contribute to the extension of the anagen phase.
Another molecule that controls the transitions between the anagen and catagen phases are vitamin D receptors.
The telogen phase coincides with major changes in gene activities. Indeed, certain regulatory proteins, such as estrogen receptors, are over-represented during this phase. The telogen phase is therefore not a completely resting phase. It may even be a crucial phase in controlling the hair cycle.
In fact, the telogen phase can be divided into a phase that is refractory to stimuli that induce follicle growth and characterized by the overregulation and activation of BMP2/4 proteins and a complementary phase where bulb stem cells become very sensitive to factors inducing the anagen phase. During this phase, the BMP signaling pathway is switched off while the Wnt/beta-catenin signaling pathway is activated and reaches an optimal level of activity at the beginning of the anagen phase.
During the telogen phase, we saw that the dermal papillae are just below the bulb. When a fairly high concentration of stem cell activators is reached, the anagen phase resumes.
Some molecules that play a role in the balance between whether or not stem cells are at rest have been identified. While stem cells seem to be kept at rest by the activation of the BMP signaling pathway in combination with the inhibition of the Wnt pathway by the TCF3 and DkkS proteins, they are activated by the inhibition of the BMP pathway and the activation of the Wnt pathway with stabilization of the beta-catenin protein:

During the early anagen phase, the bulb gradually moves away from the dermal papilla and the epithelial stem cells enter dormancy. However, the Wnt/beta-catenin signaling pathway remains active thanks to the progenitor cells (of temporary amplification) contained in the hair matrix. These cells also stabilize beta-catenin throughout the anagen phase. Higher up in the matrix, these cells stop proliferating and initiate terminal differentiations into different epithelial cell lines.
Numerous molecules that regulate the formation of the internal and external epithelial sheath have been discovered. For example, GATA3, Cutl1, and BMPs participate in the formation of the inner epithelial sheath, Sox9 and SHH participate in the formation of the outer epithelial sheath, and Wnt/beta-catenin, vitamin D receptors, vitamin D receptors, Notch, Notch, BMPs, and Foxn1 participate in the formation of the hair shaft. In contrast, TCF3 is a general inhibitor of all epithelial cell lines.
Experiments have shown that MSC-ASC exosomes cause hair growth
Exosomes contain microRNAs which, when delivered to other cells, will affect their behavior. The microRNAs contained in exosomes vary according to the stem cells from which they come. Some microRNAs will increase the proliferation of follicles while others will tend to slow them down.
The regulation of follicle growth is regulated by a paracrine mechanism in which exosomes from dermal papilla cells play an essential role.
But in addition to this type of cell, ASCs located in the fatty tissue under the dermis can also play a role in the hair cycle because ASCs differentiate into mature adipocytes that surround the hair during the transition between the telogen and anagen phase.
Numerous studies have also shown that ASCs have an effect on hair growth by increasing the proliferation of dermal papilla cells. This is because the interactions between ASC cells and dermal papilla cells are controlled by various mediators that activate the telogen — anagen transition. In particular, as has been seen, the Wnt/beta-catenin signaling pathway and the IFG-1 growth factors produced by dermal papilla cells. Exosomes derived from MSC stem cells carry a large number of growth factors and activators of Wnt/beta-catenin signaling.
The proteins contained in the exosomes of the ASCE+ HRLV product
Below are listed some proteins carried by exosomes contained in the ASCE+ HRLV product. It can be noted that a large number of them participate in the proper development of follicles:
These two groups of proteins work in synergy to promote the proliferation of keratinocyte (cells that make up the outer layer of the skin)
Alopecia and DHT
It has been seen that hair growth is governed by various endogenous factors, such as intra-cellular and inter-cellular signaling. For example, BMP and TGF signaling (in particular the factors TFG-beta1 and TGF-beta2) are known to inhibit the induction of the anagen phase, while the Wnt10b protein stimulates the transition from the telogen phase to the anagen phase. In the same way, FGF-type growth factors induce the anagen phase. Finally, other signaling pathways, when they are overactivated or, on the contrary, under-activated, play a role in the hair cycle in adults:
One of the main consequences of alopecia is the gradual reduction in the duration of the anagen phase. The follicles pass prematurely from the anagen phase to the catagen phase. Another reason is hair miniaturization, a process in which terminal solid hair is transformed into fluffy hair.
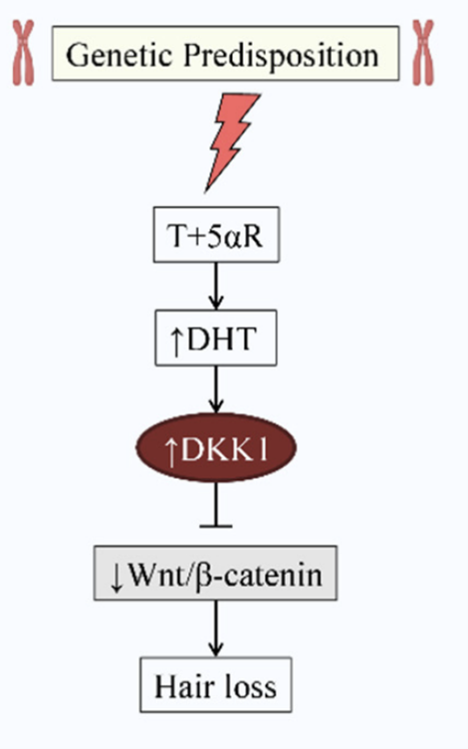
The influence of the androgen hormone DHT (dihydrotestosterone) on hair loss is well known. Androgens, including DHT, enter the follicles through the capillary network of the dermal papilla. They then attach themselves to the androgen receptors contained in the cells of the dermal papilla, and activate or deactivate certain genes. However, in people with alopecia, the dermal papilla cells contained in the follicles secrete the proteins TGF-beta1 and TGF-beta2. These inhibit the growth of epithelial cells in response to androgens.
In the body, testosterone is converted to DHT by the enzyme 5-alpha reductase. However, experiments have shown that dermal papilla cells from the follicles at the front of the scalp (which contain fewer transcriptional coactivator androgen receptors ARA70-beta/ELE1-beta but more androgen type II 5alpha reductase receptors than cells from the occipital dermal papillae - which “do not fall”), are known to secrete autocrine inhibiting factors that affect cell growth. This shows that DHT alters autocrine and paracrine processes and plays an essential role in hair loss, more so than other androgens.
Several studies have shown that under the effect of DHT, the DKK1 gene (dickkopf 1) is over-regulated in the cells of the dermal papillae of people with alopecia. This over-representation also has an impact on the growth of keratinocytes in the outer epithelial sheath.
We will see in the next section that the Dkk1 protein plays a very important role in inhibiting the proper functioning of the canonical Wnt/beta-catenin transduction pathway. The role that DHT plays in our hair can therefore be summarized by the following short diagram:
The importance of the Wnt/beta-catenin signaling pathway on hair growth
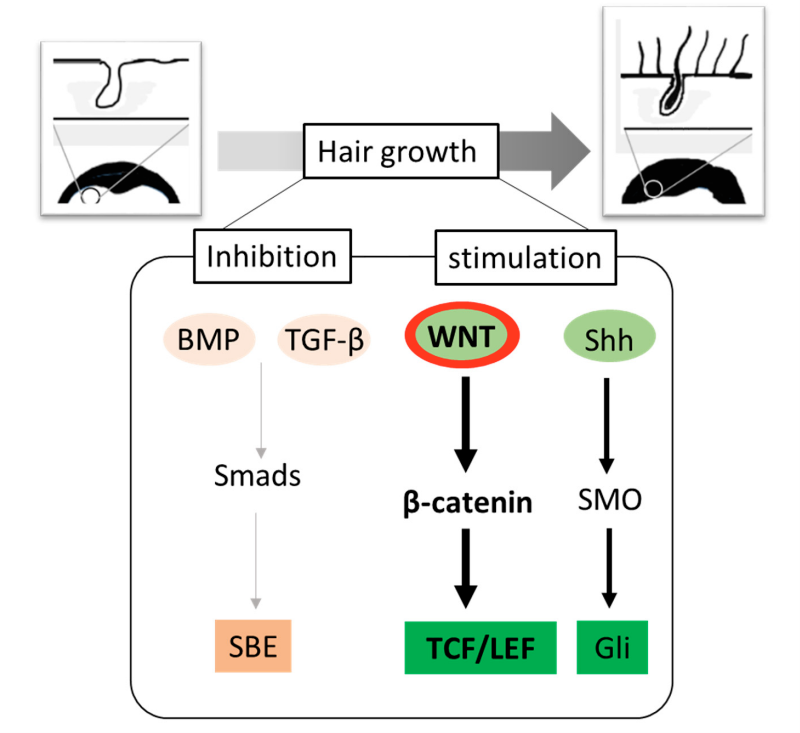
As we have seen: there are many endogenous factors that influence hair growth, including molecules involved in intercellular and extracellular signaling pathways. Activation of the anagen phase is inhibited by the activation of BMP signaling proteins and TGF-beta growth factors. On the other hand, the Wnt/beta-catenin and hedgehog (SHH) signaling pathways stimulate hair growth and the entry into the anagen phase.
We will see that in the case of the Wnt signaling pathway causing hair growth, the beta-catenin protein is stabilized in the cytoplasm of the cell and translocated into the nucleus, where it acts with proteins of the TCF/LEF family as a transcription factor. In this case, the Wnt pathway is said to be canonical. This results in the activation of genes that promote the activation of the anagen phase and therefore the growth of a new hair.
The TCF/LEF family (T-cell factor/Lymphoid Enchancer Factor) is a group of genes that encode transcription factors. They are involved in the Wnt signaling pathway, especially during the embryonic phase and stem cell development, but they also play a role in cancer and diabetes. TCF/LEF factors recruit beta-catenin as a coactivator in order to increase the probability of transcription of a specific gene. On the contrary, they can also recruit a member of the Groucho family as co-repressors (and therefore reduce the probability of gene transcription). When the betya-catenin protein is not translocated into the nucleus but destroyed in the cytoplasm of the cell, it is the protein of the Groucho family that is recruited by the TCF/LEF factors. In this case, it is said that the signaling pathway is not canonical. Unfortunately, this is what happens in people with alopecia.
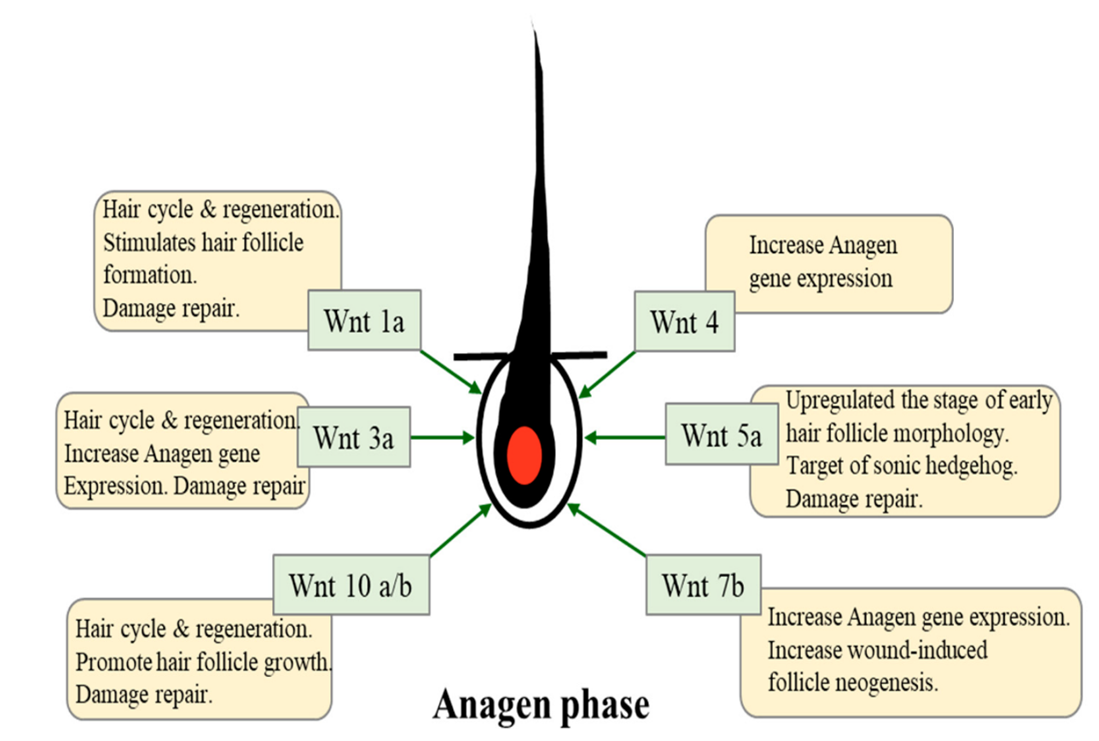
Numerous studies have shown that multiple proteins from the Wnt family are involved in the proper functioning of the hair cycle and its regeneration. For example:
Among all the signaling pathways associated with the hair cycle, many studies assume that the Wnt/beta-catenin one is the most important because DHT, which is over-regulated in alopecia, alters this signaling pathway. The main inhibitors of this signaling pathway are the proteins DKK1, SFRP2 (Secreted Frizzled-Related Protein 2) from the SFRP family and sclerotin (which binds to BMP7).
DKK1 is a powerful inhibitor of the Wnt/beta-catenin transduction pathway and experiments have shown that injections of DKK1 into the mouse scalp activates the anagen-catenin transition. This protein is over-represented in patients with alopecia and DHT interferes with the normal hair cycle in a negative way by activating the catagen phase by involving DKK1.
It is therefore reasonable to postulate that the inhibition of DKK1 is a key to promoting hair growth in people with alopecia. In addition, there are a number of microRNAs that target DKK1 and are involved in signaling pathways related to hair growth.
Let us now look in more detail at how the canonical Wnt/beta-catenin signaling pathway works and how it is deteriorated in alopecia.
The canonical Wnt signaling pathway and its deterioration in alopecia
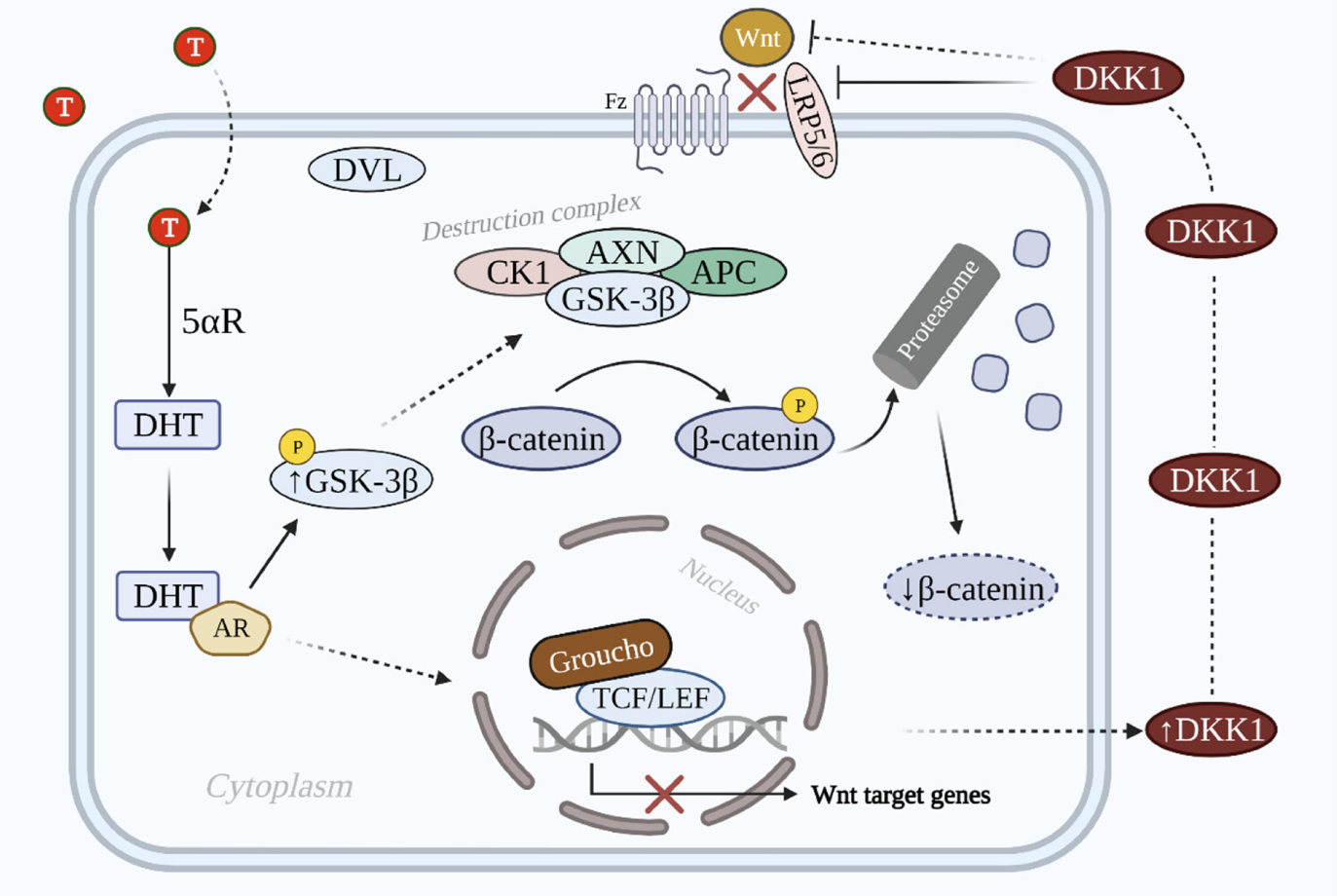
As already mentioned, the canonical Wnt signaling pathway (or Wnt/beta-catenin signaling pathway) is the Wnt signaling pathway that causes an accumulation of the beta-catenin protein in the cytoplasm of the cell and its possible translocation into the nucleus to act as a transcriptional coactivator of transcription factors belonging to the TCF/LEF family. Let's see in more detail how the DKK1 protein negatively influences this signaling pathway. canonical.
At rest, in the cytoplasm of the cell there is beta-catenin which is phosphorylated so as not to accumulate (the phosphorylation process is a chemical modification that consists in adding a phosphate group to a molecule). The destruction complex responsible for this phosphorylation process is composed of the proteins APC (Adenomatous Polyposis Coli), GSK-3alpha (Glycogen Synthase Kinase 3alpha), CK1alpha (Casein Kinase 1alpha) and PP2A (Protein Phosphate 2A). This destruction complex marks beta-catenin for degradation by the proteasome (proteasomes are enzymatic complexes that are found in the nucleus of cells and whose role is to degrade misfolded proteins — that is to say a malformed protein).
During the canonical phase of the Wnt/beta-catenin signaling pathway, Wnt ligands attach to the FZD receptor (Frizzled) and to the LRP5/P6 coreceptor. This coreceptor is transmembrane: one part is found outside the cell and another part is found in the cell's cytoplasm. As soon as Wnt binds to Frizzled and LRP5/P6, it causes the translocation of the negative Wnt regulator AXN (Axine), which binds to the tail of the LRP5/P6 protein found in the cytoplasm. The PP2A phosphate group of the destruction complex then induces the phosphorylation of the tail of the LRP5/P6 protein. This process is followed by the recruitment by phosphorylation of the phosphoprotein DVL (dishevelled) which binds to the frizzled receptor in the endodomain of the cell, which inhibits the activity of GSK-3alpha and therefore deactivates the destruction complex. This prevents the beta-catenin protein from being degraded and sent to the proteasome to be digested. Beta-catenin can then accumulate and translocate into the nucleus and then bind to TCF/LEF transcription factors. A transcriptional complex is then formed, and the transcription of a certain number of Wnt genes is then activated.
On the other hand, when the Wnt ligands do not bind to the FZD receptor, beta-catenin becomes unstable in the cytoplasm because it is phosphorylated by GSK-3alpha and CK1 since the negative regulator AXN is not translocated at the level of the LRP5/P6 tail, and therefore DVL is not recruited. GSK-3alpha is then not inhibited and the transcriptional complex is therefore not formed in the nucleus. In this case, the TCF/LEF transcription factor recruits a member of the Groucho family as a corepressor.
Studies have shown that when DKK1 proteins are over-regulated, they bind to LRP5/P6, which suppresses the Wnt signaling cascade, and inhibits follicle development, and therefore hair growth. The DKK1 protein is therefore a key factor in the development of alopecia.
Some studies also seem to show that the SFRP2 protein could inhibit the Wnt/beta-catenin signaling pathway, but this has not yet been proven. In fact, treatment with human SFRP2 recombinants on dermal papilla cells extracted from patients undergoing transplantation has considerably increased canonical Wnt signaling.

How does DHT induce DKK1
Studies have shown that the level of DHT and DKK1 are correlated. In the body, 5 alpha reductase (5alphaR) converts testosterone to DHT. This androgen hormone plays an inhibitory role on the growth of cells in the outer epithelial sheath, which induces a disturbance on normal hair growth. Experiments have also shown that anti-DKK1 drugs greatly decrease the inhibition of the growth of cells in the outer epithelial sheath. In addition, the concentration of DKK1 is higher in the scalp of people with alopecia. Finally, studies have shown that DKK1 disrupted by DHT stimulates the apoptosis of keratinocyte cells.
What are the molecules that inhibit the DKK1 protein
Some studies have looked at the effect of certain molecules on the canonical Wnt pathway. A study observed the impact of morroniside, a natural molecule found in male dogwood (sometimes called cornel or fuselier), on the canonical Wnt signaling pathway in cells of the outer epithelial sheath. The results show an increase in cell proliferation as well as an increase in beta-catenin. Another concomitant study showed that this treatment acted on DKK1.
Another natural compound, vitexin (which is found for example in passion flower), makes it possible to significantly increase the proliferation of dermal papillae in the follicles. Again, vitexin has been shown to over-regulate beta-catenin and substantially decrease the level of DKK1.
Ginseg extract also caused keratinocyte proliferation in the outer epithelial sheath, inhibited apoptosis, and revealed the effect of DKK1.
Another natural compound that activates the Wnt/beta-catenin and SHH pathway while inhibiting the TGF-beta/Smad and BMP pathways in the follicles, and therefore induces hair growth, is costunolide. It is found in the Indian costus.
The essential components of Ginkgo bilboa, Ginkolide B and Bilobalide, are also known as molecules that can help with hair growth. These molecules are associated with the activation of the Wnt/beta-catenin pathway via the inhibition of the expression of the DKK1 genes.
The antidepressant tianeptine is known to impact hair diameter, and therefore hair growth, by inhibiting DKK1. It also slows the transition from the anagen phase to the catagen phase in people with alopecia.
Finally, minoxidil also sub-regulates the production of DKK1 and TGF-beta in keratinocyte cells.
We can therefore see that a lot of studies show the influence of the inhibition of DKK1 in hair growth.
The role of microRNAs in modulating hair growth
A number of microRNAs are involved in hair growth and the deficiency of the canonical Wnt pathway impacts this growth. The idea is therefore to use certain microRNAs to regulate this pathway. I have previously focused in this report on studying the Wnt pathway, which is the most important pathway in the field of hair growth, but as already mentioned earlier, there are many other signaling pathways involved in the hair growth process.
In the following, I focused in particular on microRNAs found in exosomes from MSC stem cells and derived from dermal papillae.
A study has shown that exosomes from dermal papilla cells contain nearly 200 microRNAs. The 34 over-represented or, on the contrary, under-represented microRNAs are the following: let-7b-5p, let-7c-5p, let-7g-5p, miR-1, miR-103-3p, miR-122, miR-126-3p, miR-126-3p, miR-126-3p, miR-126-5p, miR-128-3p, miR-133a-3p, miR-145-5p, miR-148b-3p, miR-126-3p, miR-196-3p, miR-196-3p, miR-196-3p 3b-5p, miR-199c-3p, miR-199c-5p, miR-199c-5p, miR-200a, miR-200c, miR-214-3p, miR-22-5p, miR-24-3p, miR-24-3p, miR-27b-3p, miR-3b-3p, miR-320-3p, miR-330-5p, miR-330-5p, miR-3432-5p, miR-3432-5p, miR-34a, miR-243-3p, miR-243-3p, miR-423-5p, miR-423-5p, miR-423-5p, miR-423-5p, miR-423-5p, miR-3p 49a-5p, miR-451-5p, miR-499-5p, and miR-99a-5p.
This study showed that these microRNAs are involved in 40 biological functions, in particular in those related to intercellular communication occurring in follicles such as:
These 34 microRNAs potentially target 33,055 genes. The expression of some of these genes will result in an increase or decrease in the number of proteins coded. Thus, some microRNAs have a positive effect on hair growth while others will have a negative effect. This same study then studied the interaction of these 34 microRNAs with 29 genes involved in hair growth in order to identify the most represented microRNAs:
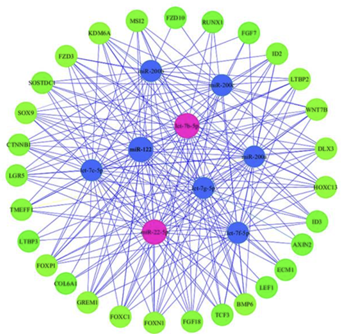
The 9 representatives are microRNAs miR-200b, miR-200c, let-7b-5p, let-7b-5p, miR-122, miR-200a, let-7g-5p, let-7c-5p, miR-5p, miR-22-5p, and let-7f-5p. Among them, the main microRNAs identified as targeting genes related to follicle growth are miR-22-5p and let-7b-5p. The study then experimentally showed that miR-22-5p inhibits the proliferation of follicle stem cells by targeting the LEF1 gene, which is involved in the canonical Wnt pathway.
Of course, other microRNAs possibly derived from other exosomes from different stem cells can have an impact on hair development. It is known that exosomes derived from MSC stem cells have a beneficial effect on hair growth. The following microRNAs are found there:
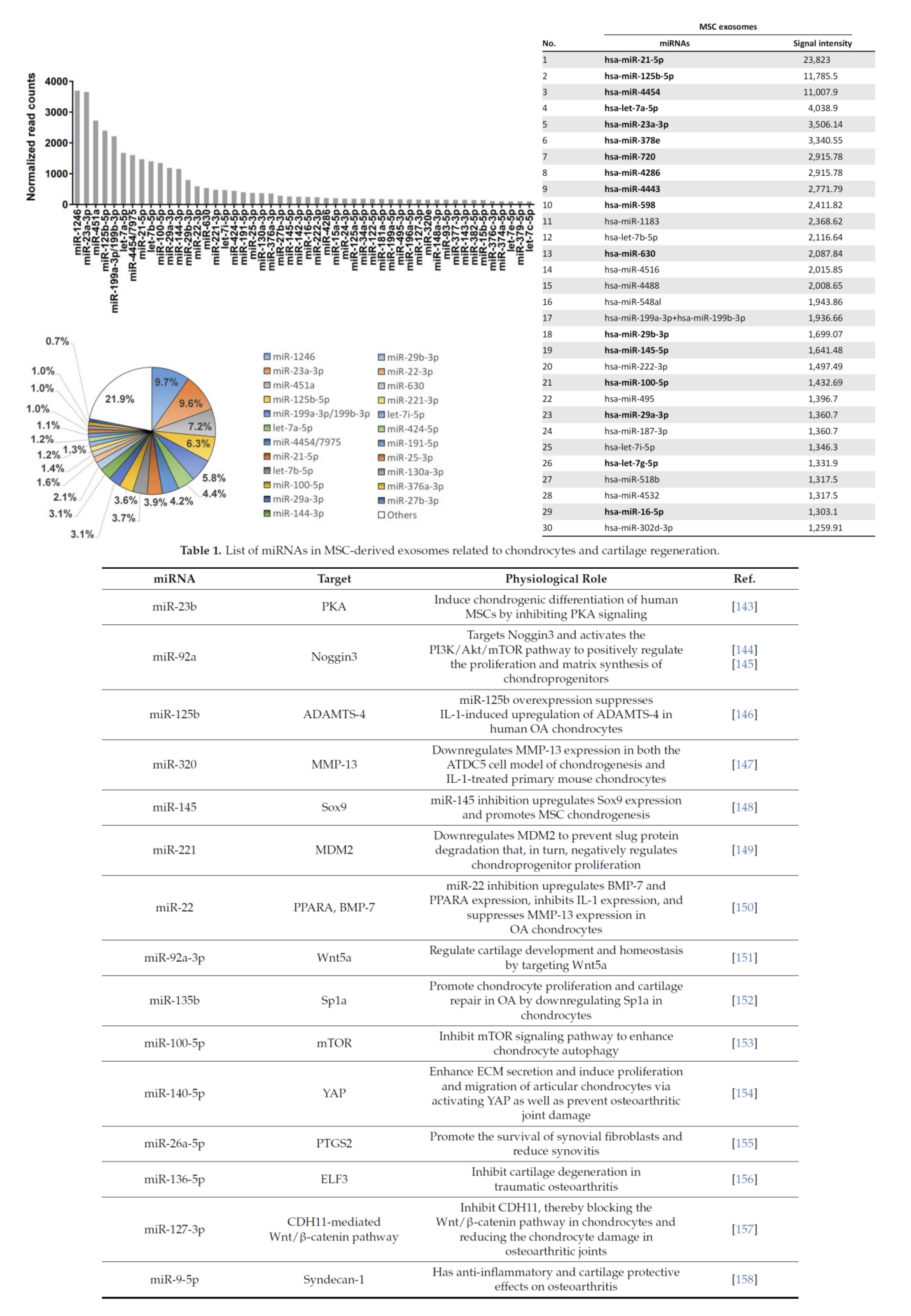
Among the microRNAs that have an impact on the regulation of DKK1, we find for example:
Examples of microRNAs that are known to have an impact on hair growth include:
It is interesting to note that even the presence of microRNAs that play a negative role in hair growth independently (such as miR-22 in MSC exosomes) does not prevent the exosomes of these stem cells from having a positive overall effect. I think that this is due to the fact that their number is lower in proportion to the other microRNAs that have a positive action and that the dynamics on which all microRNAs play is very complex. Compensation phenomena must certainly occur.
What microRNAs are contained in ASCE+ HRLV exosomes?
The question is therefore whether the microRNAs contained in the ASCE+ HRLV product can play a beneficial role in the proliferation of follicles, their maintenance in the anagen phase, or even the ability to force the hair to pass rapidly from the telogen phase to the anagen phase.
Unfortunately, the exosomes in the ASCE+ HRLV product are not exosomes extracted from dermal papilla stem cells, nor mesenchymal stem cells. The reason is that it is forbidden to sell such exosomes to the general public in certain countries (such as in Europe, the United Kingdom, the US,...), while this can be done in other countries (Korea, Japan,...), while this can be done in other countries (Korea, Japan,...).
The exosomes contained in the ASCE+ HRLV product come from rose stem cells (the English term is “Rose stem cell exosomes”). In particular, the company ExocoBio has filed the “ExoScrt” patent allowing this extraction. This makes it possible to make the use of MSC exosome derivatives accessible in certain countries where exosomes derived from human stem cells are prohibited. These exosomes have collagen-synthesizing properties and anti-inflammatory properties.
The study of these exosomes has shown that they share 904 microRNAs with those found in humans. Among these micro-RNAs, 5 are directly related to cell proliferation and three (miR-8485, miR-574-5p and mir-1246) play a role in the regulation of Wnt signaling:
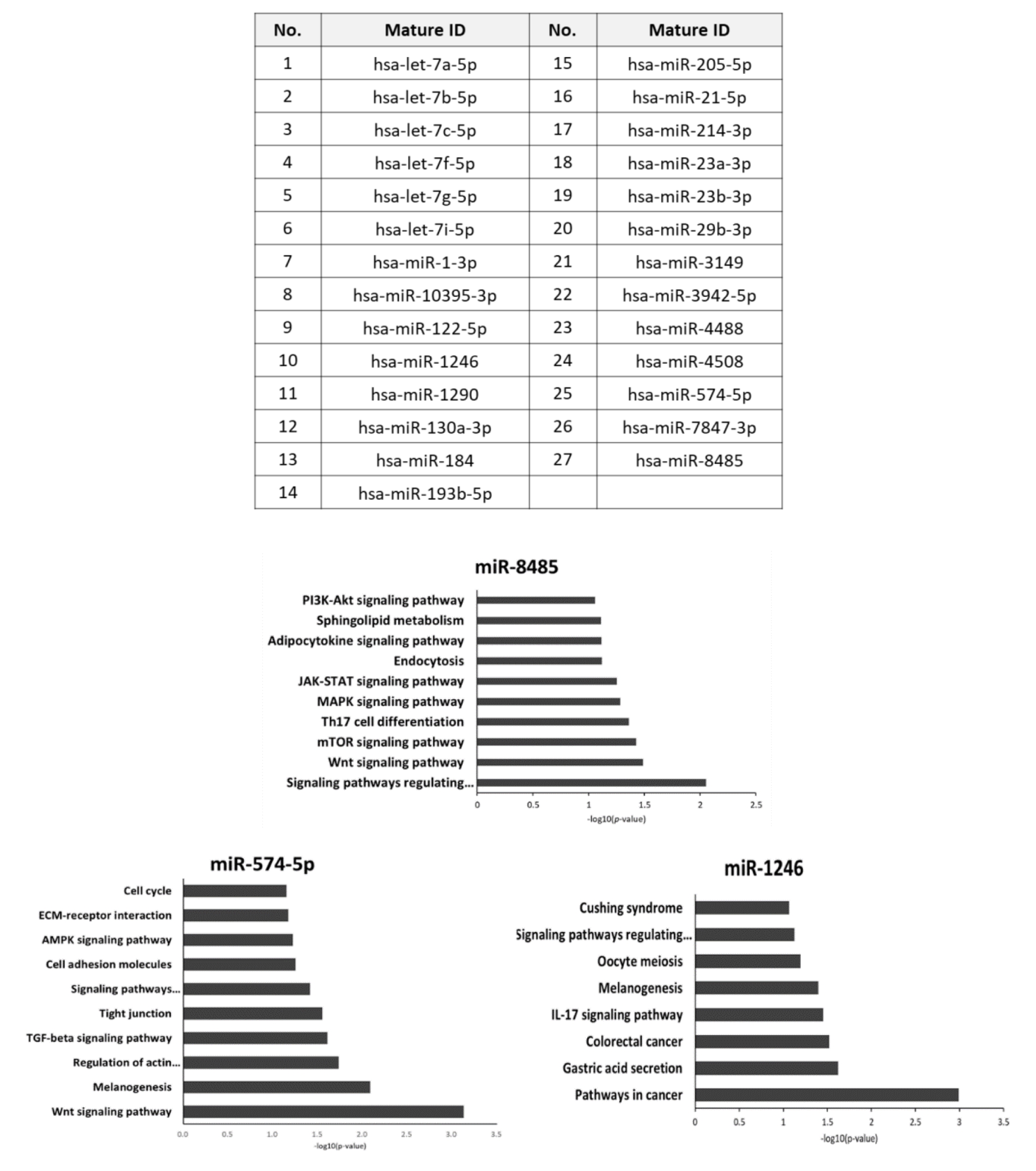
Among the microRNAs identified as having an impact on hair growth, there are a number of interesting ones:
Among those that are also found in MSC exosomes or extracts from dermal papilla stem cells are: miR-let-7a-5p, miR-let-7b-5p, miR-let-7c-5p, miR-let-7f-5p, miR-let-7f-5p, miR-let-7g-5p, miR-let-7g-5p, miR-let-7i-5p, miR-1-3p, miR-122-5p, miR-1246, miR-130a-5p, miR-1246, miR-130a-5p 3p, miR-21-5p, miR-214-3p, miR-23a-3p, miR-23b-3p, miR-29b-3p, miR-4488.
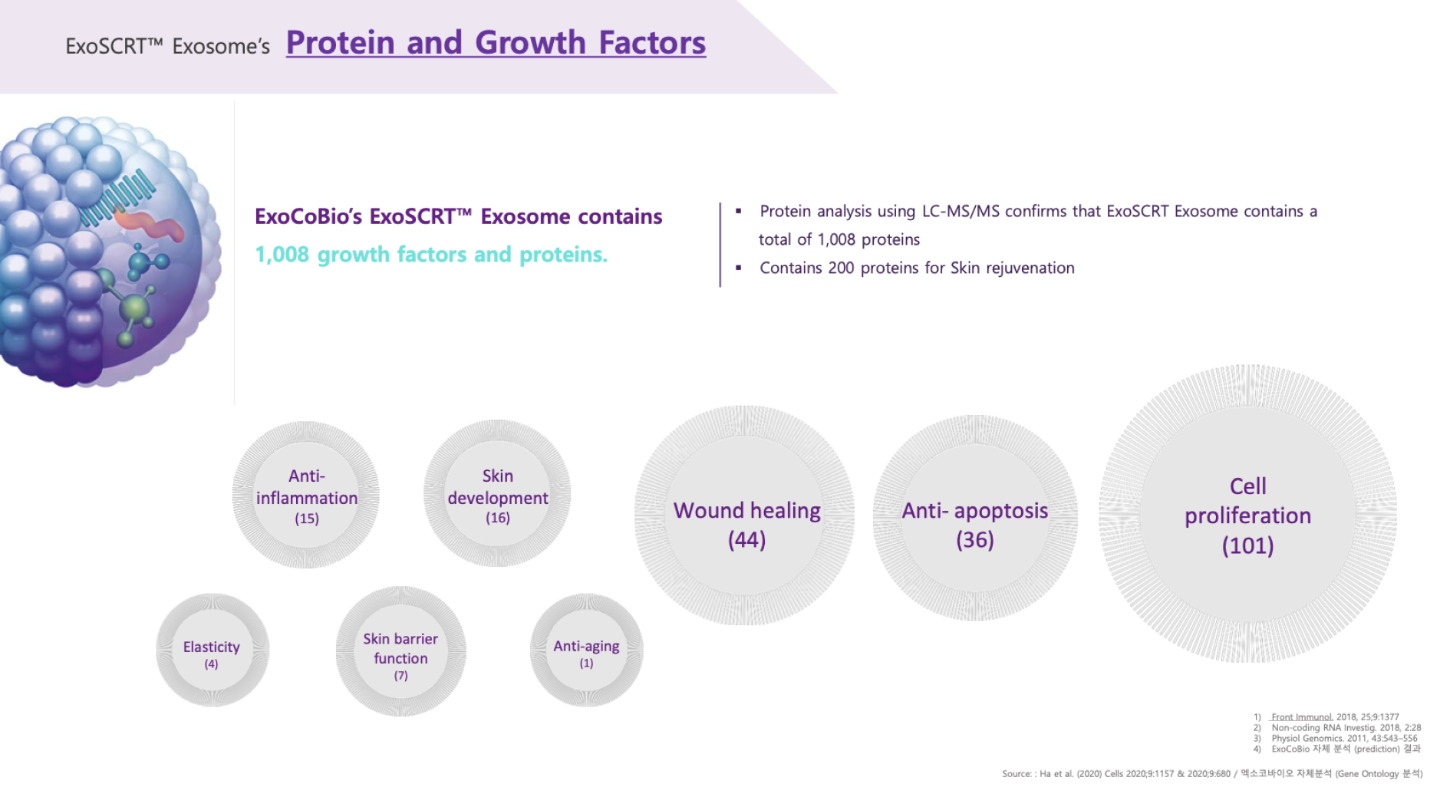
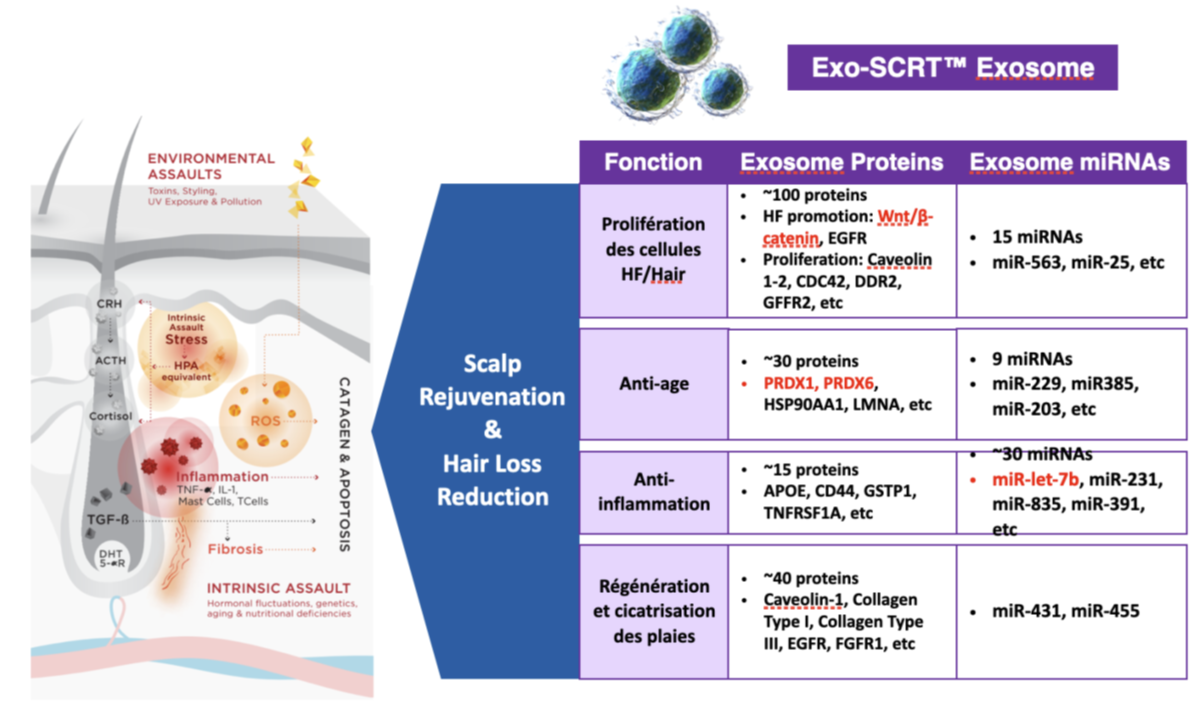
Many of the microRNAs carried in rose exosomes also have anti-aging, anti-inflammatory, and regenerative and wound healing properties. I did not study these microRNAs because I focused on those that have an impact on hair, but here is the summary given by the ExocoBio laboratory on ASCE+ HRLV exosomes:
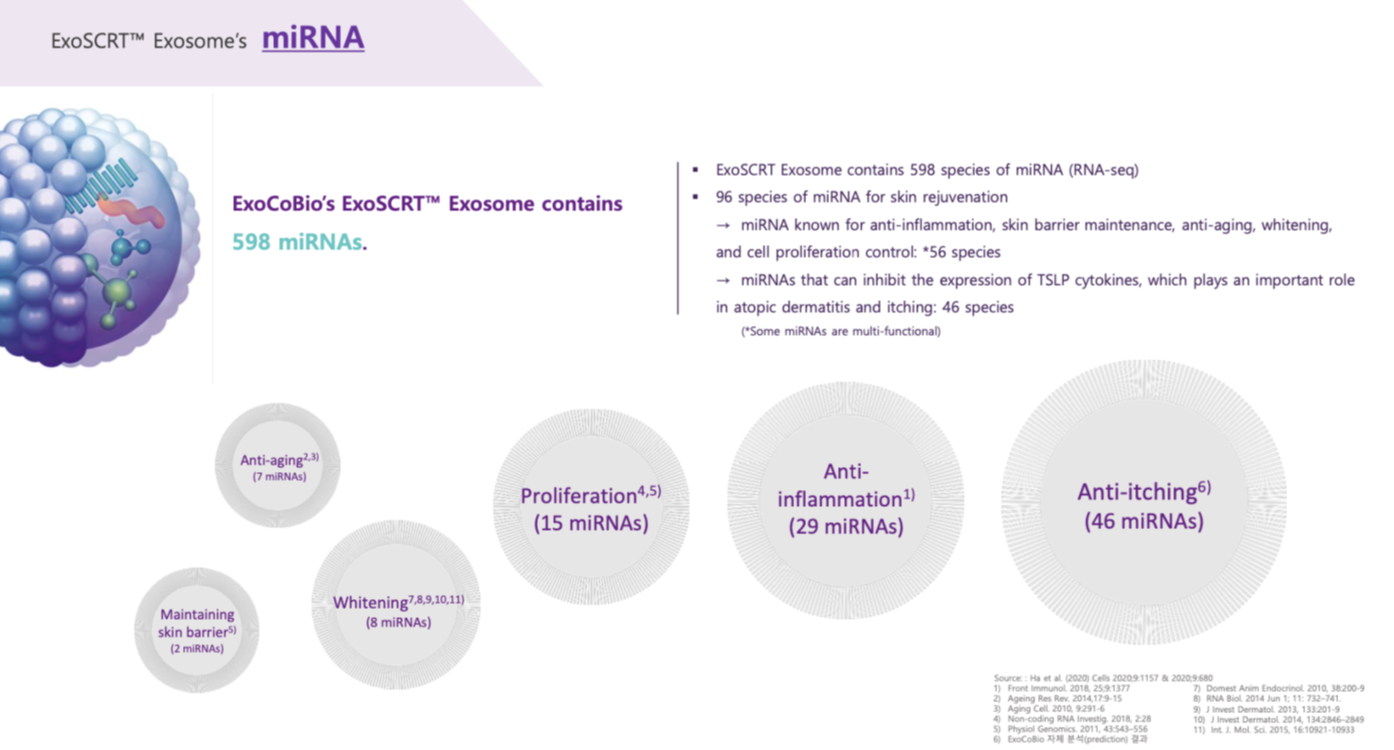
The other ingredients in the ASCE+ HRLV product
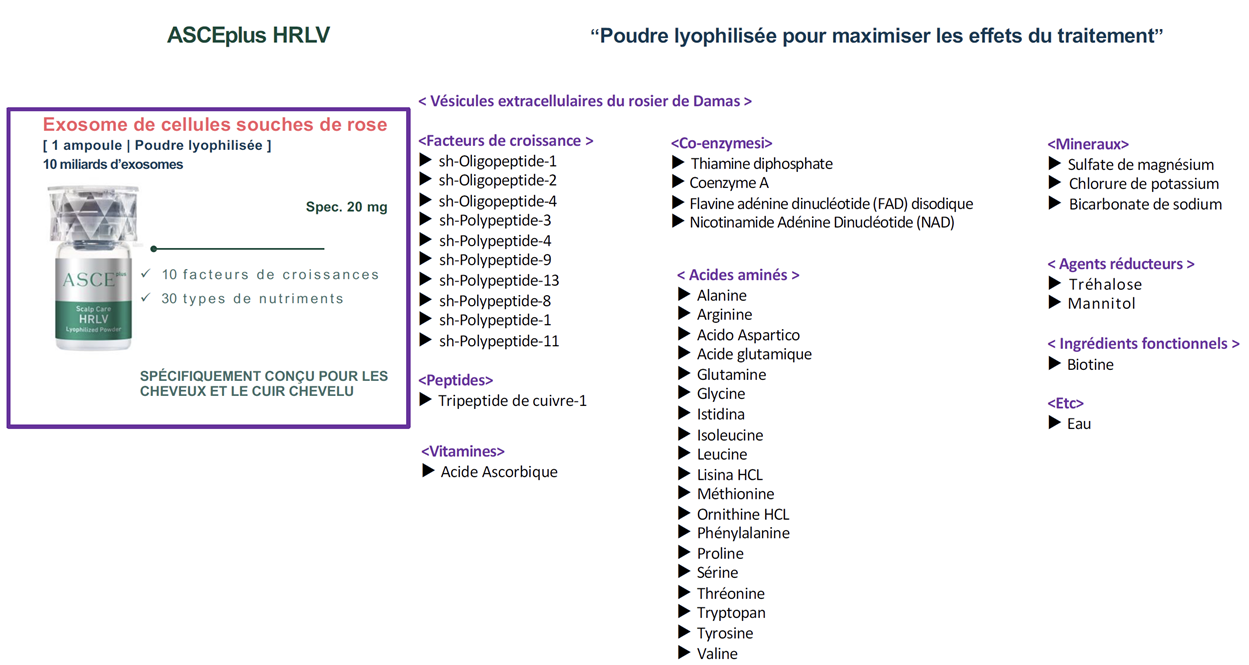
The product ASCE+ HRLV therefore seems interesting because it contains a lot of growth factors and microRNAs that have positive effects on hair growth. However, some questions remain:
I could not find the complete list of microRNAs contained in rose exosomes but the data should normally be public soon. It would be interesting to know if other microRNAs with a negative or positive action are contained in these exosomes. So I hope that this short report on exosomes will have interested you and that you (like me) will have learned some things!
Do not hesitate to contact me on the forum https://www.hairrestorationnetwork.com (my nickname is arthurSam) : Click here to send me a message !